Postdoctoral associate, Chemical Engineering, MIT
About Me
Nathan (Nat) B Wang is currently a postdoc in the Galloway Lab researching how to engineer mammalian cells, particularly in the context of cell fate reprogramming. His research interests are in developing genetic tools that work robustly in primary mammalian cells and engineering across molecular- and process- level scales. He is especially interested in working on challenges that will result in impactful cell and gene therapies.
- Mammalian synthetic biology
- Cell reprogramming
- Gene & cell therapies
PhD Chemical Engineering, 2019-2025
Massachusetts Institute of Technology
BS Chemical Engineering, Certificate in Computer Science, 2019
University of Wisconsin–Madison
I’m a postdoc in the Galloway Lab at MIT.
My work covers understanding and engineering mammalian cells for therapeutic applications, with additional expertise in the context of cell reprogramming.
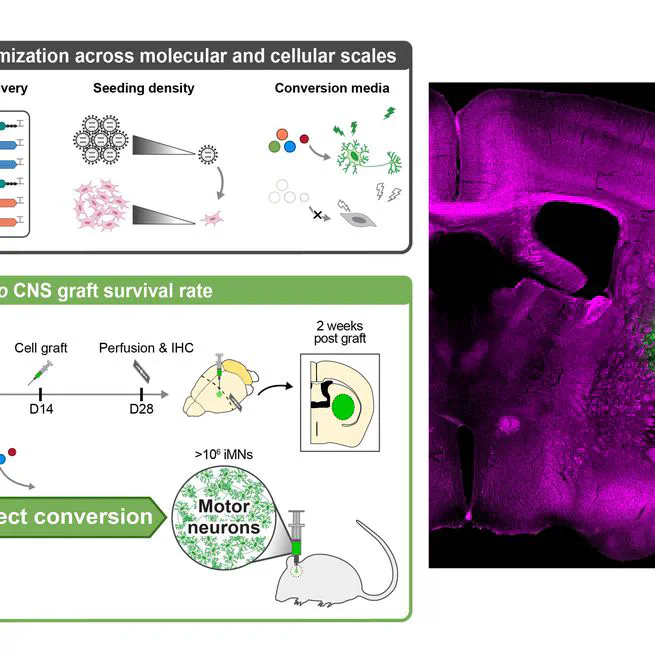
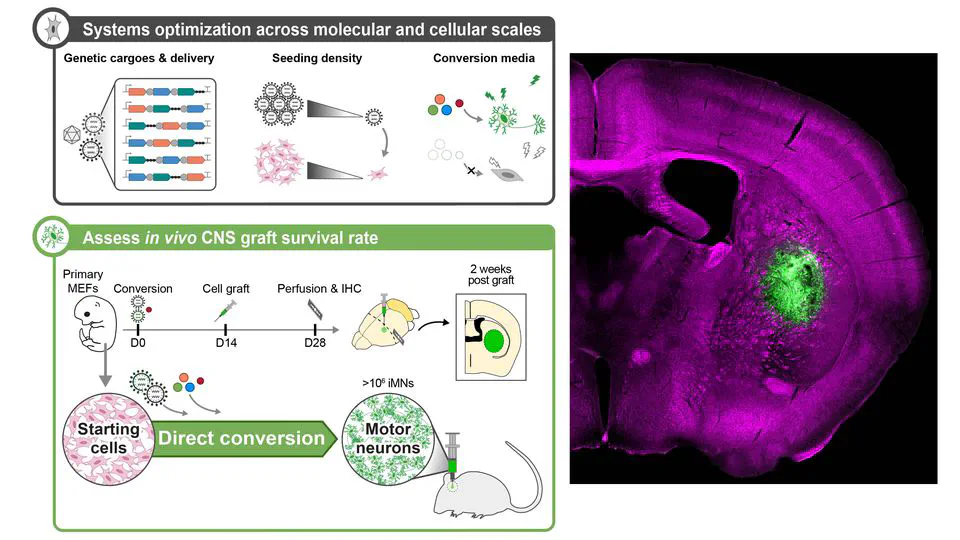
Mar 13, 2025
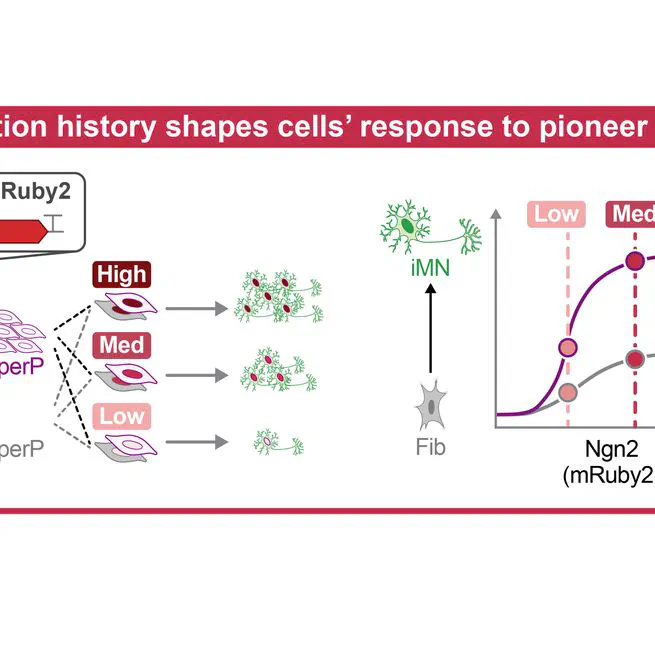
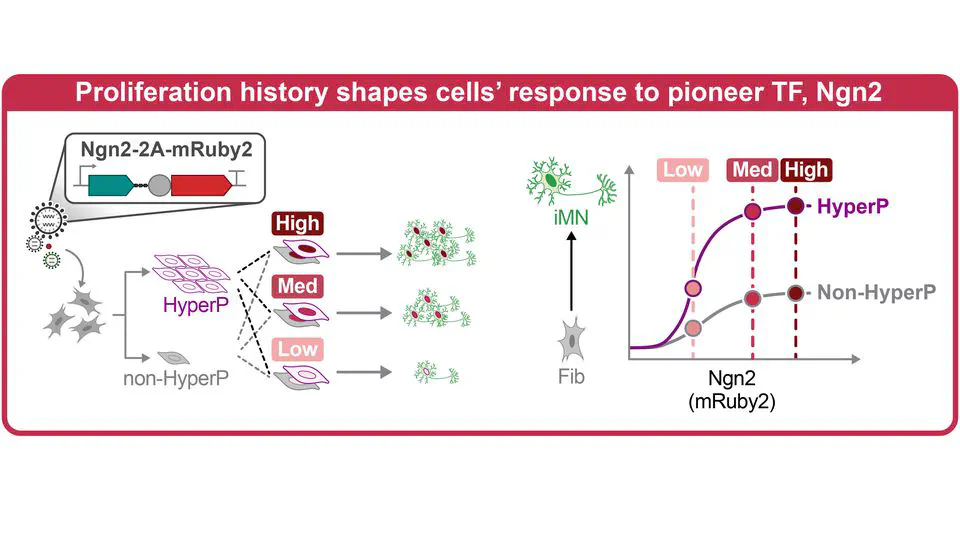
Mar 13, 2025
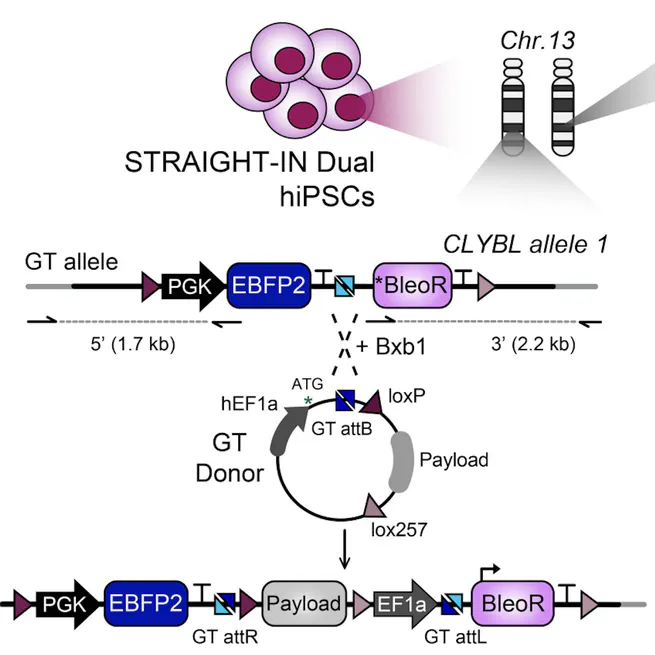
Oct 1, 2024
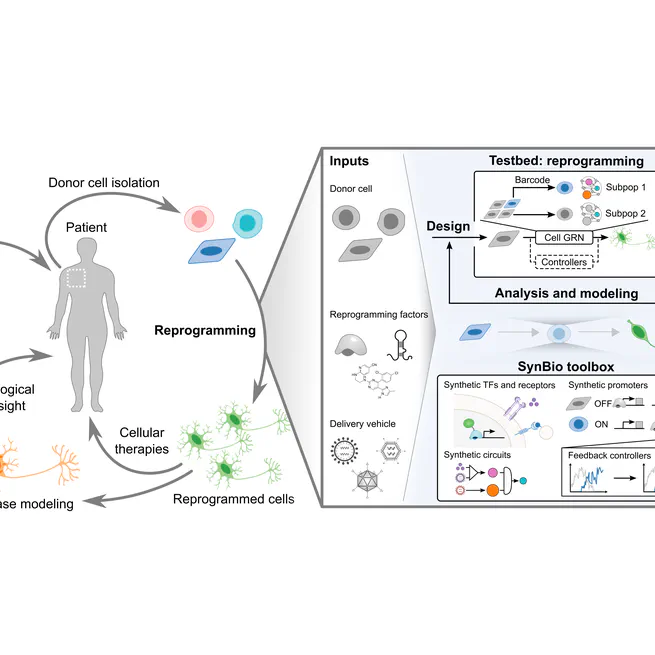
Dec 1, 2020
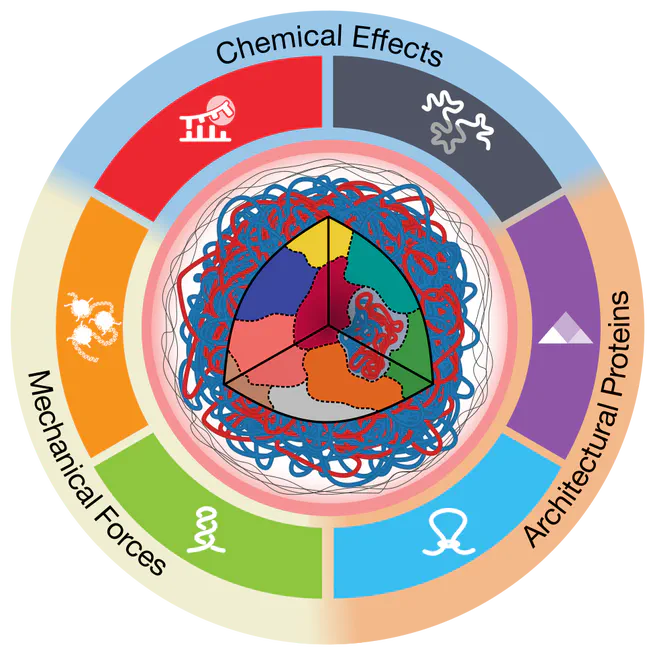
Nov 1, 2020